
Skagit Radiology Implements Ikonopedia to Modernize Breast Care Workflows
Innovative platform enables Skagit Radiology to deliver enhanced breast health services and forge stronger hospital partnerships. April 24, 2024 – Skagit Radiology has implemented Ikonopedia’s state-of-the-art platform to replace its legacy breast care workflow and reporting system, representing a strategic investment in advanced patient-centered care. This comprehensive solution equips Skagit’s radiologists with an integrated, closed-loop…

Dolbey and Ikonopedia Announce Strategic Partnership to Offer A Streamlined, Hands-Free Mammography Workflow
CINCINNATI, OH – Jan 23, 2025 – Dolbey and Company, Inc. has announced a strategic partnership with Ikonopedia, a leader in breast imaging solutions. This collaboration brings together Dolbey’s state-of-the-art speech recognition and automation technology, Fusion Narrate® powered by nVoq™, with Ikonopedia’s intuitive physician-built mammography structured reporting system. Physicians using both Fusion Narrate and Ikonopedia can navigate,…

Ikonopedia Inks Multiple Deals and Expands Service Area
Ikonopedia reaches definitive agreements with new clients Skagit Radiology and Baptist Health. Mount Sinai Health expands Ikonopedia services across Staten Island. Ikonopedia Inc., a leading provider of innovative breast imaging reporting and tracking solutions, is excited to announce the signing of a new contracts with Baptist Healthcare and Skagit Radiology. These strategic partnerships aim to…

Ikonopedia Inks Deal with Akumin Healthcare System-wide
Continued development and expansion of the Ikonopedia platform coupled with overwhelming client support sets the company apart and builds confidence in a dedicated platform for women’s healthcare. Ikonopedia, the only web-based breast imaging reporting, patient management, and MQSA Analytics platform on the market. Proudly announces the acquisition of Akumin Healthcare as a valued client. This…

A Brief Overview: National Comprehensive Cancer Network Guidelines
The National Comprehensive Cancer Network (NCCN) is a not-for-profit alliance of 33 leading cancer centers devoted to patient care, research, and education. NCCN is dedicated to improving and facilitating quality, effective, equitable, and accessible cancer care so all patients can live better lives. The NCCN offers several programs and resources to give clinicians access to tools…

Ikonopedia Announces Significant Expansion in 2023
Continued revenue growth is driven by the expansion of the installed base, increase in the overall number of patients participating in screening programs and addition of new products. Today Ikonopedia has announced that the company has exceeded expectations in all key management metrics; the company sees no end in sight for the continued rapid…
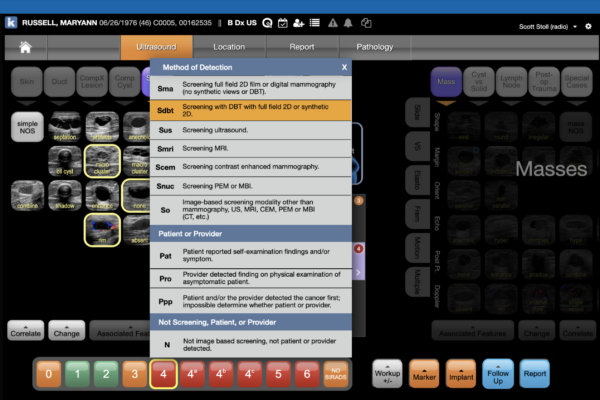
Ikonopedia Announces Implementation of the American College of Radiology “Method of Detection” Guidelines.
New functionality provides a straightforward method for radiologists to indicate the first imaging test, or sign or symptom that triggered subsequent workup and diagnosis of breast cancer. Ikonopedia is designed to streamline workflow and compliance for women’s imaging centers and radiologists. Facilitating compliance to the ACR BIRADS is a core competency of the platform and…
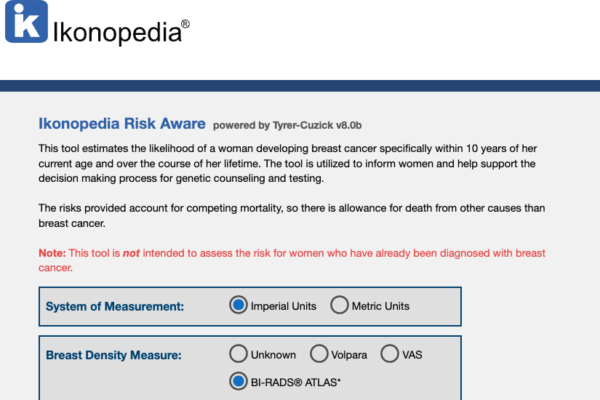
Ikonopedia Launches Risk Aware™
Ikonopedia Licenses Tyrer-Cuzick Risk Assessment Tool Enables Proactive Breast Cancer Risk Assessments for All Practices Why it Matters Breast cancer is the most commonly diagnosed malignancy in U.S. women, and the second most common cause of cancer death among women worldwide. With this knowledge, greater risk testing must be done and tools like the Tyrer-Cuzick…

Ikonopedia Announces Appointment of Brett T. Parkinson, MD as Medical Advisor
Clinical Expert Joins Ikonopedia, maker of Cloud-based, Cutting-Edge Women’s Imaging Workflow Management and Analytics Software. “We are pleased to welcome Dr. Parkinson to our team as a medical advisor,” said Emily Crane, Chief Executive Officer of Ikonopedia. “Brett’s extensive experience in women’s imaging, work with the American College of Radiology and national reputation are invaluable…

IKONOPEDIA supports SBI 2023
Just returned from National Harbor, MD where we had a wonderful time at the Society of Breast Imaging Symposium. We felt so lucky to have gotten to see so many of you in person and really enjoyed supporting this industry we love so much. We learned about interesting new focus areas and new research many…

Ikonopedia Announces Appointment of Mark Jensen to Board of Directors
Industry Veteran Brings Deep History of Innovation, Development and Success to Cutting Edge Women’s Imaging Workflow Management and Analytics Company, Ikonopedia “We are pleased to welcome Mark to our Board of Directors,” said Emily Crane, Chief Executive Officer of Ikonopedia. “Mark’s deep experience in technology, business, radiology and specifically the needs of women’s imaging services…

Novarad partners with Ikonopedia to provide radiologists the next-generation in breast imaging solutions
Novarad Corporation announces a partnership with Ikonopedia to enhance clinical excellence with a complete breast care platform, NovaMG Pro.
Social Media